Here you can find detailed information about the background and research lines of our faculty.
- Anna Menini (Full Professor) - Olfactory System
Research in Anna Menini's laboratory focuses on the olfactory system, which has become a "hot" topic in neuroscience after the Nobel Prize for Medicine and Physiology awarded in 2004 to Linda Buck and Richard Axel.
The group includes a post-doc researcher and six PhD students, who are trying to understand how odorants from the external environment bind to specific receptors, and how the signal is then transmitted to the brain.Anna Menini received her Laurea in Physics, summa cum laude, from the University of Genova, Italy, where she also obtained her PhD in Physics (Biophysics). She was a Research Associate at the Department of Physiology, Duke University Medical Center, Durham, NC, USA, then “Ricercatore” and “Primo Ricercatore” in Biophysics at the Istituto di Cibernetica e Biofisica, Consiglio Nazionale delle Ricerche, Genova, Italy. She has also been an Invited Fellow Researcher of the Japan Society for Promotion of Science at the Department of Physiological Sciences, Okazaki National Research Institutes, Okazaki, Japan. Since November 2002 she is Full Professor of Physiology at SISSA. Her research interests include sensory neuroscience and ion channels in health and disease.
RESEARCH LINES
Molecular mechanisms of olfactory transduction
Our research group is interested in understanding how the brain represents the external world. We are working in the cellular and molecular neuroscience of sensory systems, with a focus on olfaction. The olfactory system detects and discriminates among a large number of structurally diverse odorant molecules that carry information about the environment. The initial steps of olfaction occur in olfactory sensory neurons located in the olfactory epithelium of the nasal cavity.
Some animals also have a separate accessory olfactory system, whose primary sensory neurons are found in the sensory epithelium of the vomeronasal organ. We study the molecular mechanisms that transform the chemical odorant signal into the electrical messages that are transmitted to the brain.
To address these questions we use an interdisciplinary approach and the following techniques: patch-clamp electrophysiology, calcium imaging, molecular biology, immunohistochemistry and computational studies.
Calcium-activated chloride channels
We study a family of proteins called TMEM16 or anoctamins. TMEM16-A and –B have been shown to function as calcium-activated chloride channels. We use the patch-clamp technique combined with site-specific mutagenesis, immunohistochemistry and computational studies to understand the physiological role of these channels in sensory systems.
- Laura Ballerini (Full Professor) – Neurons and nanomaterials
How can an artificial material instruct neurons and influence their behavior? This is the main interest of Laura Ballerini's group, which is experimenting with new materials such as carbon nanotubes and using them as a substrate for growing neurons. Ballerini's lab has shown that carbon nanotubes can make neuronal networks more efficient, a discovery which could have important applications for neuroprosthesis and for the treatment of spinal cord lesions.
Laura Ballerini graduated (MD) at the Università di Firenze, Italy in 1988. She was a Post-doc at UCL from 1991 and later became assistant professor in Physiology at the Biophysics Sector of the International School for Advanced Studies (SISSA-ISAS) of Trieste, Italy in 1995. In 2012 LB became full professor in Physiology at the Università di Trieste, Italy and since January 2016 is full professor at SISSA. She has been working for more than 15 years on the physiology of spinal cord neurons/ networks and she has vast experience in using a variety of experimental electrophysiological techniques and in vitro model systems. LB has provided important contribution to the understanding of spinal network physiology, plasticity and development. Recently, LB has been working on the interactions between living neurons and micro-nano fabricated substrates or bioactive-composite containing carbon nanotubes. She demonstrated that carbon nanotubes substrates boost neuronal network activity by enhancing neuronal and synaptic performance. The scientific strategy at the core LB research activity is the convergence between nanotechnology, chemistry and neurobiology. Such convergence, beyond helping understand the functioning and malfunctioning of the brain, can stimulate further research in this area and may ultimately lead to a new generation of nanomedicine applications in neurology.
RESEARCH LINES
Mechanisms governing the interface between carbon based growth substrates and neurons
Study of the biophysical interactions between membranes and nanomaterials (carbon nanotubes) in neurons/nanoscaffolds hybrids, focusing on the role of membrane lipid components (rafts) in mediating adhesion and neuronal responses to conductive nanostructures.
The impact of synthetic nano-scaffolds on single cell physiology and intercellular communication modulates the complex dynamics of cell adhesion, growth and proliferation. Carbon nanotubes (CNTs) are a privileged material to support the cultivation of neurons. Available within the Ballerini’s lab is the state-of-the-art investigation showing that nanotubes can affect cell behaviour and promote attachment, growth, differentiation and long-term survival of neurons. Neurons are electrically excitable cells that transmit and process information in the nervous system. Neurons continue to grow when placed on CNTs and can still carry electrical signals when stimulated by the CNTs. As a result, several nanotube-based neural applications are being developed, such as neural prosthesis for monitoring neural activity. These achievements represent the ‘tip of the iceberg’ that only hints at the potential of CNTs for neurons. Beyond a mere interface of neuronal signalling, we reported that CNTs are able to impact the nervous system at three different levels of complexity. At the single cell level, CNTs alter the electrophysiological responses of neurons, improving their computational power. At the synaptic level, CNTs guide and induce a massive increase in synaptic density and affect the short-term dynamic of neurotransmission, strengthening synaptic signals when activated repetitively. At the level of three-dimensional spinal cord explants CNT scaffolds drive changes in neuronal fibre elongation, morphology and elastomechanical properties. Further, CNT scaffolds are able to modulate the functional performance of neurons spatially far from the scaffold. Thus, the potential of nanomaterial/neuron hybrid endeavour includes the challenge of using an artificial submicroscopic man-designed device to co-operate to neuronal network activity, generating hybrid structures able to cross the barriers between artificial devices and neurons.
Nanostructured bio-synthetic scaffolds and spinal networks
Development and multilevel application of three-dimensional biotechnological nanoscaffolds for tissue reconstruction and protection, from the simplified in vitro spinal segment to the complex, whole spinal cord system.
This project aims to exploit new concepts to obtain biomaterials for nerve tissue engineering. The goal is to develop a new generation of multifunctional implantable materials targeted to the treatment/repair of spinal cord lesion. By the convergence among different fields like nanotechnologies, polymer science and neurophysiology research, we will test nanostructured-bio-hybrid synthetic implants in cultured neuronal newtorks to address multiple key design criteria, such as biocompatibility, bioactivity, electrical conductivity, adequate rheological performance.
Growing neuronal networks with controlled 3D organization
Development of innovative platforms to artificially engineer neuronal tissues, to build customizable artificial three-dimensional neuronal networks with controlled cellular composition and networking.
This project will capitalize on state-of-the-art techniques in cell culturing and micro-nano manipulation, to envision solutions able to overcome the current limitations of standard in vitro culture methods. We wish to build artificial multidimensional cellular assembles to investigate specific properties of the DRG-spinal cord microcircuit. The customized 3D networks will allow basic research, pharmacological studies, and will open a number of possibilities to study and manipulate the properties of selective neuronal microcircuits to study, for example, sensory transmission and modulation.
Graphene based nanomaterials and excitable cells
Study of the impact of graphene-based nanomaterials on excitable cell electrical behaviour and health, assessment of biocompatibility and of the graphene/biomembrane interactions. Further developments include exploring the effects of interfacing healthy and glaucoma retinal cells.
Graphene is the material with most superlatives: it is the best conductor of heat we know, the thinnest material, it conducts electricity much better than silicon, is 100-300 times stronger than steel, has unique optical properties, it is impermeable already as a monolayer, these properties can be exploited in many areas of research; new possibilities are being recognized all the time as the science of graphene and other two-dimensional materials progresses. These properties give realistic promise of creating a new, more powerful and versatile, sustainable and economically viable technology platform based on graphene and related layered materials. As a result, graphene research has already emerged as the top research front in materials science. However, due to the unique structure of graphene, many of the possibilities it offers are still poorly understood. We will investigate the biological processes influenced by nanomaterials and nanomaterials interaction with biological membranes. Single and multiple electrophysiological measures will be used in the case of neurons and cardiomyocytes to assess the electrophysiological effects of conductive nanomaterials on electrically propagating complex tissues.
Molecular neuroscience of motoneuron disease: insights into pathology and protection mechanisms
Study of the degenerative processes in ALS model G93A organotypic spinal cultures. We study the potential involvement in motoneuron progressive degeneration of microglia-synaptic cross-talk. We will test whether SOD1 mutation improves the vulnerability of motoneurons exposed to inflammatory treatment via altering synaptic signalling in pre-motor networks.
We wish to understand the molecular mechanisms which lead to motoneuron (MN) cell loss in a genetic animal model of ALS in order to clarify at which steps, within the complex series of events underlying neurotoxicity, novel experimental approaches targeted at reducing neuronal death are best implemented. One of the major breakthroughs in beginning to understand ALS molecular pathology has been the discovery of gene mutations in the cytosolic Cu/Zn superoxide dismutase (SOD1) gene in a small proportion of all fALS patients. This discovery has led to an animal model of the disease in which the human mutations are brought to overexpression in mice. Studies based on these ALS animal models have provided new insight on the disease processes involved in selective motoneurons’ degeneration as well as on the alterations in spinal networks, ultimately linked to the SOD1 mutated enzyme expression.
- Giuliano Taccola (Associate Professor) – Spinal cord Neurophysiology
- Paul Heppenstall (Course Coordinator / Full Professor) – Physiology of somatosensation
How do neurons in the skin detect and convey information about pain, itch and touch to the brain? The Heppenstall group develops molecular, imaging and electrophysiological techniques to address this question and understand how sensory neurons give rise to our sense of touch.
Paul Heppenstall trained as a physiologist at the University of Edinburgh for his PhD, before moving to the Max Delbrueck Centrum, Berlin for postdoctoral training. In 2002, he was awarded a Junior Professor position in the Department of Anaesthesiology, Charité, Berlin where he started his own research programme in molecular pain research. In 2008, he moved to EMBL Rome where he led a research group studying the molecular physiology of somatosensation. In 2011, this was supplemented by a joint group leader position at the Molecular Medicine Partnership Unit (MMPU) in Heidelberg. Since 2018 he has been a full professor at SISSA. Paul has been working in pain research for more than 25 years and has made contributions to spinal cord physiology and pharmacology, sensory mechanotransduction, and peripheral nervous system biology. A major focus of his laboratory is to now translate research findings into new treatments for pain and itch.
RESEARCH LINES
Dissecting sensory neuron cell diversity
The peripheral nervous system is made up of subpopulations of functionally diverse neuronal types that detect and encode different types of stimuli. In order to understand this functional organization, the Heppenstall laboratory has developed a number of molecular genetic tools with which to manipulate peripheral neurons. This includes Cre driver lines to target distinct populations of neuron, and Cre reporter lines that allow for the probing of function through imaging and chemogenetic ablation. These technologies have enabled the lab to understand how distinct sensations such as itch versus touch are generated, and to investigate how injury triggers reorganization of the system provoking chronic pain.
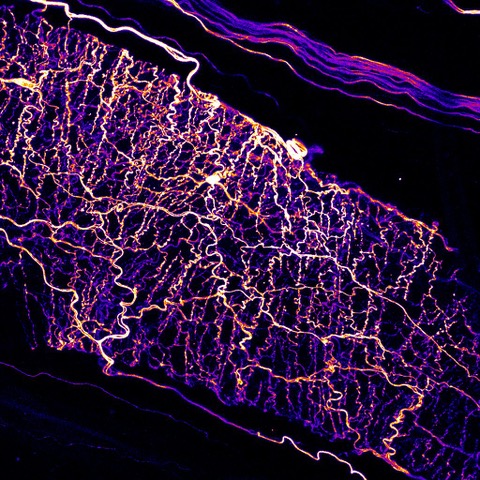
Sensory neurons surrounding a blood vessel in the skin. Credit: Barenghi
Targeting sensory neurons using ligands
Based upon their work on sensory neuron diversity, the Heppenstall group has been developing pharmacological approaches with which to gain control of sensory neuron activityand inhibit pain or itch at its source. At the core of the technology, the group has engineered protein ligands which bind selectively to subtypes of neuron that provoke itch, mechanical hypersensitivity or inflammatory pain. Using these ligands they have been able to direct photosensitizers to neurons in the skin and achieve long-term inhibition of itch and pain. The lab is now exploring how these ligands can be used to deliver small molecules, proteins or genes into neurons, thus allowing for precise control over neuronal function.
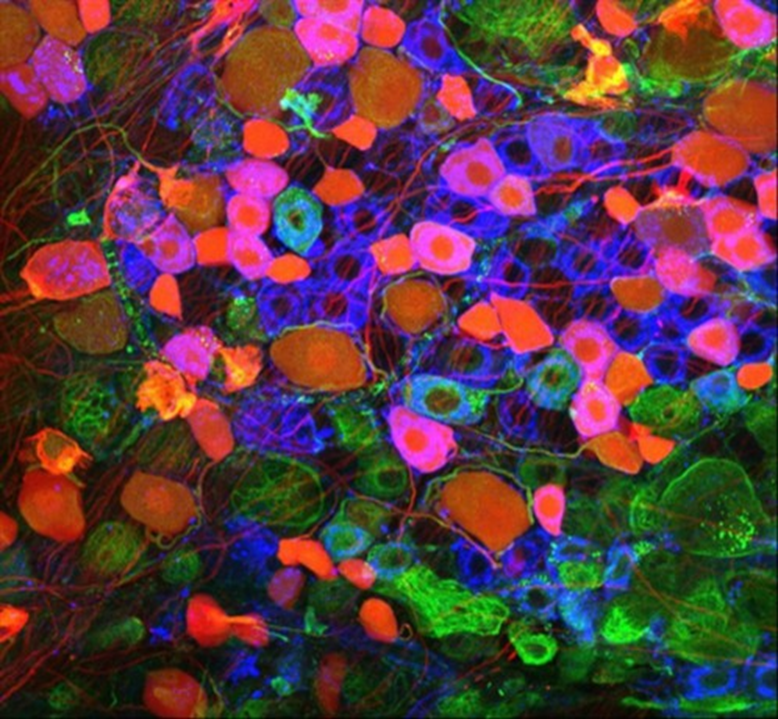
Gene delivery in sensory neurons. Credit: Barenghi
Tubulin acetylation and touch
Acetylated microtubules play a critical role in regulating the sensitivity of sensory neurons to mechanical touch. The protein Atat1 is the enzyme responsible for acetylation of α-tubulin, and in its absence mice, flies and nematode worms display a profound loss of mechanical sensitivity but no other overt phenotype. The Heppenstall group generated the first mouse knockout of Atat1, and demonstrated that mice lacking α-tubulin acetylation display a profound loss of mechanical touch and pain. They are now exploiting this finding to develop small molecule inhibitors for tubulin acetylation as a novel class of analgesic
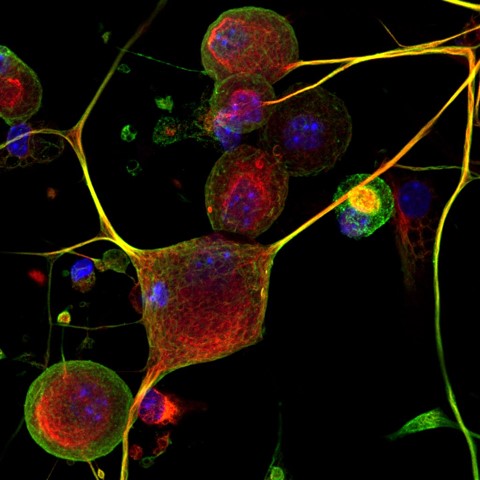
Acetylated microtubules in sensory neurons. Credit: Castaldi
Imaging sensory neurons
The Heppenstall group has a long-standing interest in developing methods for live imaging of neurons in the skin. They have focused on chemogenetic approaches that utilize genetic targeting to localize synthetic indicators to cells of interest. For example, the lab produced the first Cre dependent reporter mouse line expressing the SNAP-tag, and demonstrated that it allowed for bothmanipulation of behaviour and monitoring of cellular fluorescence in complex tissue. More recently, they have generated a semisynthetic tethered voltage indicator called STeVI1 that can be genetically targeted to neuronal membranes for optical monitoring of voltage. In ongoing work, the team are optimizing this technology to visualize how neuronal circuitry is activated by defined sensory inputs.
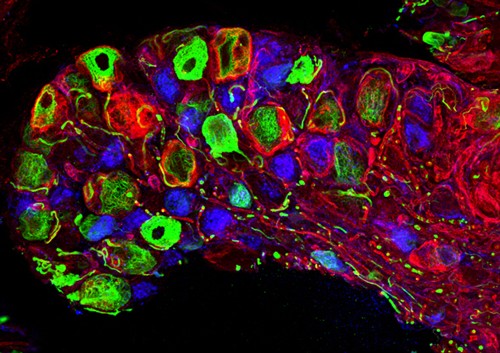
Snap-tag labelling of sensory neurons. Credit: Castaldi
Genetic sensory diseases
The Heppenstall laboratory has a strong interest in developing technology to treat heritable sensory diseases which cause itch and pain. They have been working on a rare skin disease called familial primary localized cutaneous amyloidosis (FPLCA) that provokes severe itching and damage to the skin. The group generated a mouse model of FPLCA and found that by targeting a photosensitizer to itch sensing neurons they were able to reverse symptoms of the disease for several months. They are now exploring whether disease causing mutations in FPLCA and other genetic diseases can be corrected in the skin through cell-directed gene editing.
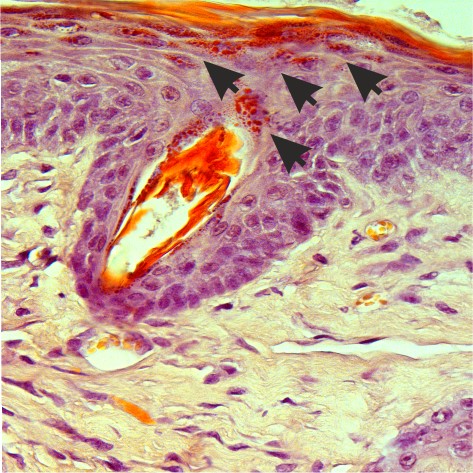
Amyloid deposits in the skin of an FPLCA mouse. Credit: Nocchi
- Michele Giugliano (Associate Professor) - From neurons to networks
MG graduated in Electronic Engineering in 1997 at the University of Genova, Italy, and in 2001 received a PhD in Bioengineering and Computational Neuroscience from the Polytechnic of Milan, Italy. He was then awarded by the Human Frontiers Science Program Organisation with a long-term fellowship to pursue postdoctoral research in cellular electrophysiology at the Institute of Physiology of the University of Bern (Switzerland), working with Prof. Hans-Rudolf Luescher and Prof. Stefano Fusi. In 2005, he became junior group-leader in the experimental lab of Prof. Henry Markram at the Brain Mind Institute of the Swiss Federal Institute of Technology of Lausanne.
In 2008, he was appointed faculty at the University of Antwerp (Belgium) where he became full professor in 2016, taking over the Theoretical Neurobiology lab founded by Prof. Erik De Schutter and extending its scope to interdisciplinary research in experimental neuroscience and neuroengineering. During 2013-2015, he was visiting scientist at the Neuroelectronics Flanders Institute at IMEC, Leuven and also served other visiting academic positions at the Swiss Federal Institute of Technology of Lausanne and at the University of Sheffield, UK.
Since 2019, he has been Principal Investigator at SISSA and director of the Neuronal Dynamics Laboratory.
RESEARCH ACTIVITIES
The research interests of MG include
- quantifying information processing and the response properties of cortical neurons;
- investigating the impact of synaptic "noise" and ion channels "noise";
- developing mathematical models of neurons and neuronal networks;
- investigating heterogeneities in human and rodent cortical microcircuits;
My academic ResearchID and ORCID profiles are publicly accessible.
Our PLoS Biology paper has been featured on Nature Podcast!
Internship, Theses, Monographic & PhD Projects
A number of monographic projects and theses are available in the Neuronal Dynamics Laboratory, throughout each academic year. These are aimed at students of Life Sciences, Neuroscience, Physics, and Computer Sciences curricula, and cover topics of cellular electrophysiology, neurobiology, biophysics, theoretical and computational modelling and simulation, image processing, neuroinformatics, data-bases, robotics, analog-digital acquisition and real-time operating system.
Interested? You are welcomed to get in touch for an appointment, to know more about our activities and about how you could contribute to it before, within, or beyond a master thesis project.
We are always searching for talented, curios, and passionate PhD students to train, as well as for creative thinkers and independent-minded postdoctoral researchers to collaborate with. In case of mutual interest, we help in preparing grant applications to national and international funding agencies such as JSPS, HFSPO, Boehringer Ingelheim, EC, and other sources. Students from Brazil may benefit from the Science Without Borders program, with application deadlines at the end of January, May and September. Students from China might monitor the calls from the China Scholarship Council.In particular, potential postdoctoral scientists may wish to consider applying for support from Marie Curie Actions (deadline in August), HFSP (deadline in August), EMBO (deadlines in mid-February and mid-August).
Click here for our lab website, publications, news, videos..
- Katja Reinhard (Assistant Professor) - Flexibility in Circuits & Behaviour
Katja Reinhard studied biomedical sciences at the Universities of Fribourg and Bern, Switzerland. During her PhD in the lab of Thomas Münch at the Center for Integrative Neuroscience in Tübingen, Germany, she trained as a physiologist and studied the retina of various species (mice, pigs, humans). She then moved to Leuven, Belgium, to study how visual information from the retina is processed and routed by the superior colliculus to guide innate behaviours as a postdoc in the lab of Karl Farrow. During her postdoc, Katja became an expert in viral tracing and in-vivo electrophysiology, and she set up a new research line to study the evolution of circuits underlying innate behaviours. In November 2022, Katja joined SISSA as a new assistant professor and head of the ‘Flexibility in Circuits & Behaviour’ lab.
RESEARCH ACTIVITIES
Imagine cycling through a town and a car door suddenly opens in front of you. You might react by hitting the breaks or by dodging the door and cycling around it. Which reaction is induced depends on external and internal factors – the traffic next to you, your stress level because of the meeting you're cycling to…
Avoiding danger, such as the car door, is one of the most essential and conserved set of behaviors, observed in most species from crabs to primates. To optimize an animal’s survival, the type, magnitude, and kinetics of avoidance responses need to be flexible and adaptable to the current context (traffic, stress…). However, the neural circuit elements that allow for this flexibility in behavioural output are largely unknown. Our aim is to identify how information about the environment and state can adapt behavioural decision making. To achieve this, we employ viral tracing methods, in-vivo electrophysiology and calcium imaging as well as behavioural assays in various rodent species.
- Associated members
Loredana Casalis – Scientist at Elettra Sincrotrone – AFM litography
Dan Cojoc – Senior Scientist at IOM-CNR
- Recent publications
05/03/2024 J. Neurosci. Methods
Mapping lumbar efferent and afferent spinal circuitries via paddle array in a porcine model
A.G. Steele, G. Taccola, A.M. Frazier, M. Manzella, M. Hogan, P.J. Horner, A.H. Faraji, D.G. Sayenko
13/02/2024 ACS Nano
Hazel Lin, Tina Buerki-Thurnherr, Jasreen Kaur, Peter Wick, Marco Pelin, Aurelia Tubaro, Fabio Candotto Carniel, Mauro Tretiach, Emmanuel Flahaut, Daniel Iglesias, Ester Vázquez, Giada Cellot, Laura Ballerini, Valentina Castagnola, Fabio Benfenati, Andrea Armirotti, Antoine Sallustrau, Frédéric Taran, Mathilde Keck, Cyrill Bussy, Sandra Vranic, Kostas Kostarelos, Mona Connolly, José Maria Navas, Florence Mouchet, Laury Gauthier, James Baker, Blanca Suarez-Merino, Tomi Kanerva, Maurizio Prato, Bengt Fadeel, Alberto Bianco
03/02/2024 Life
Aurora Santin, Beatrice Spedicati, Alessandro Pecori, Giuseppe Giovanni Nardone, Maria Pina Concas, Gioia Piatti, Anna Menini, Giancarlo Tirelli, Paolo Boscolo-Rizzo, Giorgia Girotto
25/01/2024 BioRxiv
Dual effects of ARX poly-alanine mutations in human cortical and interneuron development
Vanesa Nieto-Estevez, Parul Varma, Sara Mirsadeghi, Jimena Caballero, Sergio Gamero-Alameda, Ali Hosseini, Sonal Goswami, Marc J. Silvosa, Drew M. Thodeson, Zane R. Lybrand, Michele Giugliano, Christopher Navara, Jenny Hsieh
23/1/2024 Molecular Brain
The brain cytokine orchestra in multiple sclerosis: from neuroinflammation to synaptopathology
Roberta Amoriello, Christian Memo, Laura Ballerini, Clara Ballerini
23/1/2024 Cellular and Molecular Life Sciences
Ribosomal protein L24 mediates mammalian microRNA processing in an evolutionarily conserved manner
Yonat Tzur, Serafima Dubnov, Nimrod Madrer, Adi Bar, Bettina Nadorp, Nibha Mishra, Paul Heppenstall, Estelle R. Bennett, David S. Greenberg, Katarzyna Winek, Hermona Soreq
29/10/2023 Exp. Neurol.
Giuliano Taccola, Roger Kissane, Stanislav Culaclii, Rosamaria Apicella, Wentai Liu, Parag Gad, Ronaldo M. Ichiyama, Samit Chakrabarty, V. Reggie Edgerton
09/09/2023 Sci Adv
Pathway-specific inputs to the superior colliculus support flexible responses to visual threat
Chen Li, Norma K. Kühn, Ilayda Alkislar, Arnau Sans-Dublanc, Firdaouss Zemmouri, Soraya Paesmans, Alex Calzoni, Frédérique Ooms, Katja Reinhard, Karl Farrow
05/07/2023 bioRxiv
The neural basis of defensive behaviour evolution in Peromyscus mice
Felix Baier, Katja Reinhard, Victoria Tong, Julie Murmann, Karl Farrow, Hopi E. Hoekstr
03/07/2023 bioRxiv
Spinal facilitation of descending motor input
Giuliano Taccola, Roger Kissane, Stanislav Culaclii, Rosamaria Apicella, Wentai Liu, Parag Gad, Ronaldo M. Ichiyama, Samit Chakrabarty, V. Reggie Edgerton
14/09/2023 Chem. Eur. J.
Lorenza Tortella, Irene Santini, Neus Lozano, Kostas Kostarelos, Giada Cellot, Laura Ballerini
21/07/2023 iScience
Andres Hernandez-Clavijo, Cesar Adolfo Sánchez Triviño, Giorgia Guarneri, Chiara Ricci, Fabian A Mantilla-Esparza, Kevin Y Gonzalez-Velandia, Paolo Boscolo-Rizzo, Margherita Tofanelli, Pierluigi Bonini, Michele Dibattista, Giancarlo Tirelli, Anna Menini
04/05/2023 Sci Rep
Passive limb training modulates respiratory rhythmic bursts
Rosamaria Apicella and Giuliano Taccola
30/04/2023 Biomolecules
Belén Cortés-Llanos, Rossana Rauti, Ángel Ayuso-Sacido, Lucas Pérez, Laura Ballerini
06/02/2023 Chem Senses
Paradoxical electro-olfactogram responses in TMEM16B knock-out mice.
Giorgia Guarnieri, Simone Pifferi, Michele Dibattista, Johannes Reisert, Anna Menini
02/02/2023 Cell Mol Neurobiol
Suprapontine Structures Modulate Brainstem and Spinal Networks.
Atiyeh Mohammadshirazi, Rosamaria Apicella, Benjamín A. Zylberberg, Graciela L. Mazzone and Giuliano Taccola
02/12/2022 ACS Appl. Nano Mater.
Cellot G, Jaquemin L, Reina G, Franceschi Biagioni A, Fontanini M, Chaloin O, Nishina Y, Bianco A, Ballerini L.
14/06/2022 Experimental Neurology
Stochastic spinal neuromodulation tunes the intrinsic logic of spinal neural networks.
Taccola G, Ichiyama RM, Edgerton VR, Gad P.
24/06/2022 bioRxiv
Mohammadshirazi A, Apicella R, Taccola G.
25/01/2022 Int. J. Mol. Sci.
Frisari S, Santo M, Hosseini A, Manzati M, Giugliano M, Mallamaci A.
21/10/2022 ACS Appl. Mater. Interfaces
TEGylated Double-Walled Carbon Nanotubes as Platforms to Engineer Neuronal Networks.
Myriam Barrejón, Francesca Zummo, Anastasiia Mikhalchan, Juan J. Vilatela, Mario Fontanini, Denis Scaini, Laura Ballerini, and Maurizio Prato
02/09/2022 Front Neuroanat
No evidence for age-related alterations in the marmoset retina.
Haverkamp S, Reinhard K, Peichl L, Mietsch M.
12/08/2022 Sci Adv
Thalhammer A, Fontanini M, Shi J, Scaini D, Recupero L, Evtushenko A, Fu Y, Pavagada S, Bistrovic-Popov A, Fruk L, Tian B, Ballerini L.
04/08/2022 Nanoscale
2D MXene interfaces preserve the basal electrophysiology of targeted neural circuits.
Xiao M, Li X, Pifferi S, Pastore B, Liu Y, Lazzarino M, Torre V, Yang X, Menini A, Tang M.
Cell Death Dis. 2022 Aug 13;13(8):705. doi: 10.1038/s41419-022-05144-6.PMID: 35963860
Spelat R, Jihua N, Sánchez Triviño CA, Pifferi S, Pozzi D, Manzati M, Mortal S, Schiavo I, Spada F, Zanchetta ME, Ius T, Manini I, Rolle IG, Parisse P, Millán AP, Bianconi G, Cesca F, Giugliano M, Menini A, Cesselli D, Skrap M, Torre V.
eNeuro. 2022 May 17;9(3):ENEURO.0471-21.2022. doi: 10.1523/ENEURO.0471-21.2022. Print 2022 May-Jun.PMID: 35487703
Sarno N, Hernandez-Clavijo A, Boccaccio A, Menini A, Pifferi S.
Sci Rep. 2022 Jul 6;12(1):11447. doi: 10.1038/s41598-022-15572-1.PMID: 35794236
Expression pattern of Stomatin-domain proteins in the peripheral olfactory system.
Gonzalez-Velandia KY, Hernandez-Clavijo A, Menini A, Dibattista M, Pifferi S.
Cell Physiol Biochem. 2022 Jun 7;56(3):254-269. doi: 10.33594/000000531.PMID: 35670331
Hernandez-Clavijo A, Gonzalez-Velandia KY, Rangaswamy U, Guarneri G, Boscolo-Rizzo P, Tofanelli M, Gardenal N, Sanges R, Dibattista M, Tirelli G, Menini A.
Cellular, Molecular, Physiological, and Behavioral Aspects of Spinal Cord Injury, Academic Press, 2022, Pages 51-63
Neuromodulation and restoration of motor responses after severe spinal cord injury.
Sayenko DG, Cerrel Bazo HA, Horner PJ, Taccola G.

